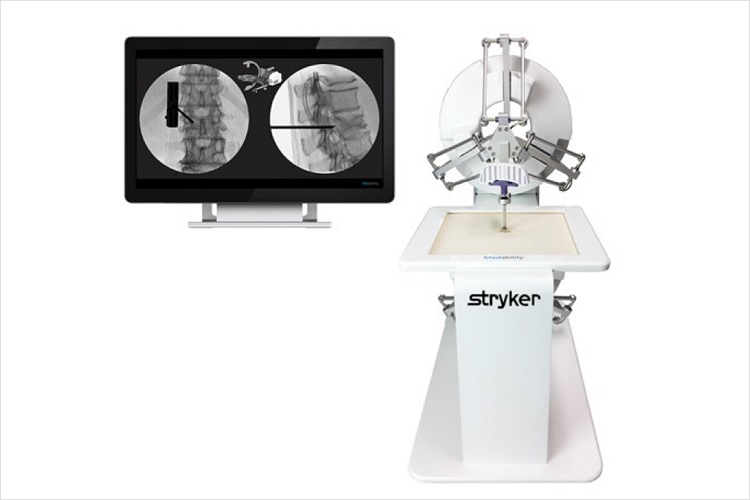
The DuO simulator is designed to support MISS (minimally invasive spine surgery) training through an innovative approach, which makes surgical training cost-effective, easy, safe and flexible.
Each clinical training case is simulated using high-end robotics with an extremely realistic haptic sensation.
The training offers unique features:
- Haptic feedback experience
The DuO simulator system combines the force feedback of two high-end robots using the DualForce technology which provides realistic haptic feedback for bone simulations in the market. The simulator creates notably different feedbacks for the skin, soft tissue, cortical and trabecular bone, which have been thoroughly tested and validated by independent orthopedic surgeons. - Precise and robust
The instrument is tracked by the robotic devices which provide for simulations with submillimeter precision, independent of the lines of sight of navigational systems or of metallic interferences occurring with magnetic tracking systems. Therefore, the DuO System allows for a quick set up in any kind of environment, be it the office, hotel room, operating theater or training center.
Interested? This is a training opportunity for you and your team.
Stryker offers the chance to book the DuO Simulator System for on-site training with selected vertebral augmentation devices (SpineJack system, AVAflex, iVAS Elite) to practice treating vertebral fractures and placing pedicle screws.
Please contact charlotte.schuetz@stryker.com or michaela.felsch@stryker.com to arrange an appointment.
The above information was provided by Medability GmbH, the manufacturer of the DuO-simulator (http://www.medability.de).
______________________________
This document is intended solely for the use of healthcare professionals. A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that healthcare professionals be appropriately trained in the use of any particular product before use. The information presented is intended to demonstrate the breadth of Stryker product offerings. A healthcare professional must always refer to the package insert, product label and/or Instructions for Use before using any Stryker product. Products may not be available in
all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in your area. Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Stryker. All other trademarks are trademarks of their respective owners or holders. The products depicted are CE marked in accordance with applicable EU Regulations and Directives. SMACC Project No. 2022-33973Copyright © 2022 Stryker www.stryker.com